Shelton Iron and Steel Co | Shelton Bar | Earl
Granville Works
![]()
How it works: Coke Production for Blast Furnace Ironmaking
| back to 'How the plant works' |
INTRODUCTION
Blast furnace operation demands the highest quality of raw materials, operation, and operators. Coke is the most important raw material fed into the blast furnace in terms of its effect on blast furnace operation and hot metal quality. A high quality coke should be able to support a smooth descent of the blast furnace burden with as little degradation as possible while providing the lowest amount of impurities, highest thermal energy, highest metal reduction, and optimum permeability for the flow of gaseous and molten products. Introduction of high quality coke to a blast furnace will result in lower coke rate, higher productivity and lower hot metal cost.COKE PRODUCTION
The cokemaking process involves carbonization of coal to high temperatures (1100°C) in an oxygen deficient atmosphere in order to concentrate the carbon. The commercial cokemaking process can be broken down into two categories: a) By-product Cokemaking (as used at Shelton) and b) Non-Recovery/Heat Recovery Cokemaking.By-product Coke Production:
The coke produced at Shelton works came from wet-charge, by-product coke oven batteries (Figure 1). The entire cokemaking operation is comprised of the following steps: Before carbonization, the selected coals from specific mines are blended, pulverized, and oiled for proper bulk density control. The blended coal is charged into a number of slot type ovens wherein each oven shares a common heating flue with the adjacent oven. Coal is carbonized in a reducing atmosphere and the off-gas is collected and sent to the by-product plant where various by-products are recovered. Hence, this process is called by-product cokemaking.
|
|
 Figure 2: Incandescent coke in the oven waiting to be "pushed". |
|
COKE PROPERTIES
High quality coke is characterized by a definite set of physical and chemical properties that can vary within narrow limits. The coke properties can be grouped into following two groups: a) Physical properties and b) Chemical properties.a) Physical Properties:
Measurement of physical properties aid in determining coke behavior both inside and outside the blast furnace (Figure 3). In terms of coke strength, the coke stability and Coke Strength After Reaction with CO2 (CSR) are the most important parameters. The stability measures the ability of coke to withstand breakage at room temperature and reflects coke behavior outside the blast furnace and in the upper part of the blast furnace. CSR measures the potential of the coke to break into smaller size under a high temperature CO/CO2 environment that exists throughout the lower two-thirds of the blast furnace. A large mean size with narrow size variations helps maintain a stable void fraction in the blast furnace permitting the upward flow of gases and downward of molten iron and slag thus improving blast furnace productivity.
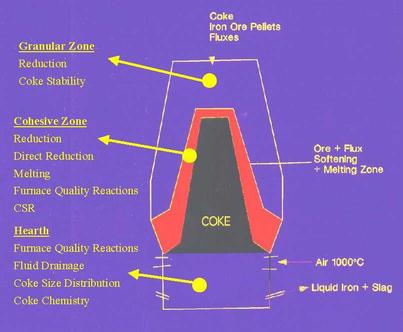
Blast Furnace Operating Zones and Coke Behaviour.
FACTORS AFFECTING COKE QUALITY
A good quality coke is generally made from carbonization of good quality coking coals. Coking coals are defined as those coals that on carbonization pass through softening, swelling, and resolidification to coke. One important consideration in selecting a coal blend is that it should not exert a high coke oven wall pressure and should contract sufficiently to allow the coke to be pushed from the oven. The properties of coke and coke oven pushing performance are influenced by following coal quality and battery operating variables: rank of coal, petrographic, chemical and rheologic characteristics of coal, particle size, moisture content, bulk density, weathering of coal, coking temperature and coking rate, soaking time, quenching practice, and coke handling. Coke quality variability is low if all these factors are controlled. Coke producers use widely differing coals and employ many procedures to enhance the quality of the coke and to enhance the coke oven productivity and battery life.
Based on an article by Hardarshan S. Valia, Scientist, Ispat Inland Inc
questions/comments/contributions?
email: Steve Birks![]()